A) trigonal pyramidal
B) tetrahedral
C) bent
D) linear
E) trigonal planar
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is an sp2 hybridized carbon?
A) ![]()
B) ∙ CH3
C) ![]()
D) A and B
E) A, B and C
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Give the number of nonbonding pairs of electrons in H2NOH.
A) 0
B) 1
C) 2
D) 3
E) 4
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Each lone pair of electrons on the O atom in methanol (CH3OH) occupies a(n) ________ orbital.
A) s
B) p
C) sp
D) sp2
E) sp3
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Essay
BF3 has a dipole moment of zero. Propose a structure for BF3 that is consistent with this information.
Correct Answer

verified
BF3 is trig...View Answer
Show Answer
Correct Answer
verified
View Answer
Short Answer
What kind of molecular orbital (σ, σ*, π, or π*)results when the two atomic orbitals shown below interact in the manner indicated? 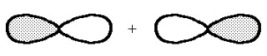
Correct Answer

verified
Correct Answer
verified
Essay
Why is the C-H bond in ethene (H2C  CH2)shorter and stronger than the C-H bond in ethane (CH3CH3)?
CH2)shorter and stronger than the C-H bond in ethane (CH3CH3)?
Correct Answer

verified
The length and strength of a C-H bond de...View Answer
Show Answer
Correct Answer
verified
View Answer
Essay
Expand the condensed structure below to show the covalent bonds and the lone-pair electrons. (CH3)2CHCH2CHO
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is closest to the C-O-C bond angle in CH3-O-CH3?
A) 180°
B) 120°
C) 109.5°
D) 90°
E) 160°
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Essay
Give the hybridizations of the carbons, from left to right, in CH3CH  CHCl.
CHCl.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which carbon(s) in the following molecule is (are) sp hybridized? 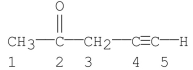
A) carbon 1
B) carbon 2
C) carbons 1, 3
D) carbons 4
E) carbons 4, 5
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Essay
Draw a Lewis structure for the molecule given and show all formal charges. CH2CO
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many carbon-carbon sigma bonds are in the molecule shown? 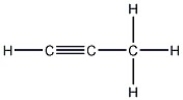
A) 1
B) 2
C) 3
D) 4
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Essay
Provide the mathematical equation for the dipole moment of a bond, and identify the variables.
Correct Answer

verified
μ = e × d, where μ is the bond...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Identify the hybridization of carbon in H2CO.
A) sp
B) sp2
C) sp3
D) sp4
E) s3p
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Essay
Give the hybridization, shape, and bond angle for each carbon in CH3CN.
Correct Answer

verified
CH3 - sp3, te...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Give the formal charge on nitrogen in NH4.
A) -2
B) -1
C) 0
D) +1
E) +2
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The lone-pair electrons of the methyl anion occupy a(n) ________ orbital.
A) s
B) p
C) sp
D) sp2
E) sp3
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Short Answer
What kind of molecular orbital (σ, σ*, π, or π*)results when the two atomic orbitals shown below interact in the manner indicated? 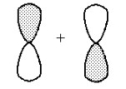
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which bond in the following molecule is the shortest? 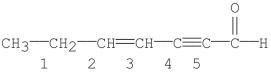
A) bond 1
B) bond 2
C) bond 3
D) bond 4
E) bond 5
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Showing 41 - 60 of 81
Related Exams