A) C2p + H1s
B) C2sp + H1s
C) C2sp2 + H1s
D) C2sp3 + H1s
F) A) and B)
Correct Answer

verified
Correct Answer
verified
True/False
The following two structural formulas represent isomers. 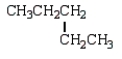
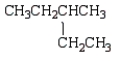
B) False
Correct Answer

verified
Correct Answer
verified
Essay
Draw bond-line structures of all of the primary (1 ) alcohols that have the formula C5H12O.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is a tertiary (3 ) alcohol? 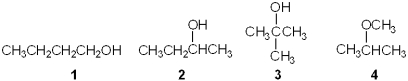
A) 1
B) 2
C) 3
D) 4
F) All of the above
Correct Answer

verified
Correct Answer
verified
True/False
The maximum number of electrons that a molecular orbital can contain is four.
B) False
Correct Answer

verified
Correct Answer
verified
Essay
Provide a neatly drawn figure to show the atomic orbitals that overlap to form each of the bonds in water (H2O) and which contain the lone pair of electrons. Label each orbital with its hybridization.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following molecules has a molecular dipole moment? 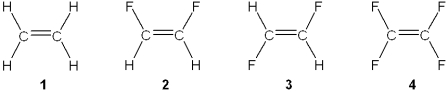
A) 1
B) 2
C) 3
D) 4
F) A) and C)
Correct Answer

verified
Correct Answer
verified
True/False
The most electronegative elements in the periodic table are generally found toward the right in a horizontal row and toward the top in a column.
B) False
Correct Answer

verified
Correct Answer
verified
Essay
Circle all of the sp hybridized atoms in the following molecular structure. 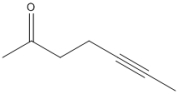
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which atomic orbitals overlap to form the carbon-hydrogen bonding molecular orbitals of ethane, CH3CH3?
A) C2p + H1s
B) C2sp + H1s
C) C2sp2 + H1s
D) C2sp3 + H1s
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following elements has the highest electronegativity?
A) N
B) C
C) O
D) S
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the ground-state electronic configuration of a fluorine atom (fluorine: atomic number 9) ?
A) 1s12s12p7
B) 1s22s22p5
C) 1s22s22p6
D) 1s02s22p7
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the ground-state electronic configuration of a sodium cation (sodium: atomic number 11) ?
A) 1s22s22p63s1
B) 1s22s22p53s1
C) 1s22s22p6
D) 1s22s22p63s2
F) None of the above
Correct Answer

verified
Correct Answer
verified
Essay
Draw bond-line structures of all of the alcohols that have the formula C4H10O.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the approximate strength of the C-C bond of ethane?
A) 376 kJ/mol (90 kcal./mol)
B) 422 kJ/mol (101 kcal./mol)
C) 556 kJ/mol (133 kcal./mol)
D) 727 kJ/mol (174 kcal./mol)
F) A) and B)
Correct Answer

verified
Correct Answer
verified
True/False
The following molecules all contain the same functional group except 2. CH3OH CH3OCH3 CH3CH2OH CH3CH(OH)CH3 1 2 3 4
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Rank the following in order of decreasing importance as a contributing resonance structure to the molecular structure of acetone, CH3COCH3 (more important > less important) 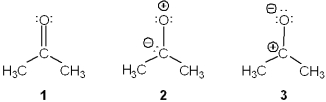
A) 1 > 2 > 3
B) 1 > 3 > 2
C) 2 > 1 > 3
D) 3 > 1 > 2
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following best represents the shape of a 2p atomic orbital of carbon? 
A) 1
B) 2
C) 3
D) 4
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following bonds is the most polar?
A) F-F
B) H-F
C) C-H
D) C-Si
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements is not true about the acetate anion, CH3CO2-?
A) The oxygen atoms bear the same amount of charge
B) The two carbon-oxygen bonds are the same length
C) The carbon atom bears the negative charge
D) It is basic
F) All of the above
Correct Answer

verified
Correct Answer
verified
Showing 41 - 60 of 115
Related Exams