A) NH3
B) H2O
C) HCl
D) CH4
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is the strongest acid?
A) HCl
B) HI
C) HF
D) HBr
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Short Answer
Provide the equation that relates the equilibrium constant, Keq, to the acid dissociation constant, Ka, for the following equilibrium.

Correct Answer

verified
Correct Answer
verified
Essay
Provide the equation for the acid dissociate constant, Ka,for the following equilibrium.

Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following equations is correct?
A) DG = DH - TDS
B) DH = DG - TDS
C) DG = DH - DS
D) DG = DH - DS /T
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Essay
Provide a brief explanation, based on features of the molecules, for the following trend in pKa values?
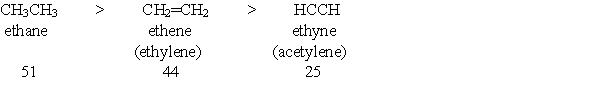
Correct Answer

verified
This trend is best understood in terms o...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Which of the following has a pKa value of approximately 25?
A) CH3CH3
B) CH2=CH2
C) HCºCH
D) CH3CH2OH
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is the strongest acid?
A) CH3OH
B) CH3CHO
C) CH3COCH3
D) CH3COOH
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is a Lewis acid but not a Brønsted-Lowry acid?
A) CH3COOH
B) AlCl3
C) H2O
D) CH3OH
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the value of the equilibrium constant, Keq, for the following reaction? 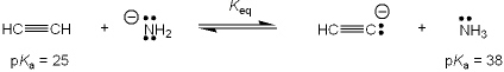
A) 1013
B) 10-13
C) 13
D) (1/13)
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Essay
Provide a brief explanation, based on features of the molecules, for the following trend in pKa values?

Correct Answer

verified
This trend is best understood in terms o...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
What is the approximate pKa value of HCl?
A) -7
B) 5
C) 16
D) 51
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following anions is the strongest base?
A) CH3COO-
B) HO-
C) NH2-
D) Cl-
F) None of the above
Correct Answer

verified
Correct Answer
verified
Essay
What is the value of the equilibrium constant for the following equilibrium?
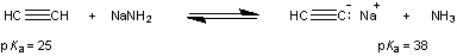
Correct Answer

verified
log10Keq = pKa(acid) - pK...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Which atom in the following structure is preferentially protonated by a strong acid? 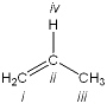
A) i
B) ii
C) iii
D) iv
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is present in the highest concentration upon dissolution of H2SO4 in water?
A) H2SO4
B) H+
C) H3O+
D) HO-
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds has the highest pKa?
A) SiH4
B) H2S
C) PH3
D) HCl
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is a feature of a Brønsted-Lowry acid?
A) proton donor
B) proton acceptor
C) electron pair donor
D) electron pair acceptor
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is a definition of the rate-determining step of a reaction mechanism?
A) the first step
B) the last step
C) the step that crosses the highest energy barrier
D) the most exothermic step
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following terms describes the role of ethyne in the acid-base reaction shown? 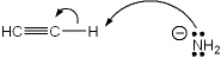
A) Brønsted-Lowry acid
B) Brønsted-Lowry base
C) Lewis acid
D) Lewis base
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Showing 41 - 60 of 75
Related Exams