A) 1
B) 2
C) 3
D) 4
F) B) and C)
Correct Answer

verified
A
Correct Answer
verified
Multiple Choice
Which of the following terms describes the reactivity of trimethylamine, (CH3) 3N?
A) Brønsted-Lowry acid and Lewis acid
B) Brønsted-Lowry base and Lewis base
C) Lewis acid and not a Brønsted-Lowry acid
D) Lewis base and not a Brønsted-Lowry base
F) None of the above
Correct Answer

verified
B
Correct Answer
verified
Multiple Choice
Which of the following terms describes the role of ethanol in the acid-base reaction shown? 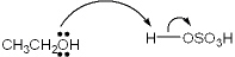
A) Brønsted-Lowry acid
B) Brønsted-Lowry base
C) Lewis acid
D) Lewis base
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is a feature of a Lewis acid?
A) proton donor
B) proton acceptor
C) electron pair donor
D) electron pair acceptor
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the approximate pKa value of acetic acid?
A) -7
B) 5
C) 16
D) 51
F) A) and D)
Correct Answer

verified
B
Correct Answer
verified
Multiple Choice
Which of the following terms describes the reactivity of boron tribromide, BBr3?
A) Brønsted-Lowry acid and Lewis acid
B) Brønsted-Lowry base and Lewis base
C) Lewis acid and not a Brønsted-Lowry acid
D) Lewis base and not a Brønsted-Lowry base
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is the strongest base?
A) NaOH
B) NaCO3
C) H2O
D) CH3OH
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Essay
Use curved arrows to show the movement of pairs of electrons in the following reaction between a Lewis acid and a Lewis base, and show the structure of the product.
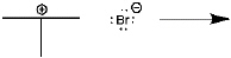
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is easiest to deprotonate?
A) CH4
B) CH3CH3
C) CH2=CH2
D) HCºCH
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which species is the conjugate acid in the following acid-base reaction? 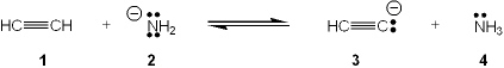
A) 1
B) 2
C) 3
D) 4
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which set of curved arrows accounts for the deprotonation of an acid (A-H) by a base (:B) ? 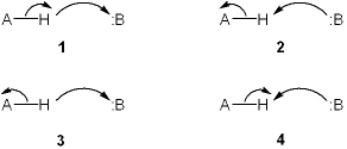
A) 1
B) 2
C) 3
D) 4
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following has a pKa value of approximately 16?
A) HBr
B) CH3COOH
C) CH3CH2OH
D) HCºCH
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following concepts can be used to rationalize the observation that acetic acid is a stronger acid than methanol?
A) electronegativity
B) resonance
C) valence shell electron pair repulsion theory
D) Pauli exclusion principle
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is present in the highest concentration upon dissolution of acetic acid in water?
A) OH-
B) H3O+
C) CH3COOH
D) CH3COOH+
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds has the lowest pKa?
A) H2O
B) H2S
C) H2Se
D) H2Te
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is a definition of the activation energy of a reaction?
A) the difference in Gibbs free energy between the reactants and the transition state
B) the difference in Gibbs free energy between the reactants and the intermediate
C) the difference in Gibbs free energy between the reactants and the product
D) the difference in Gibbs free energy between the transition state and the product
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Essay
What is the value of the equilibrium constant for the following equilibrium?
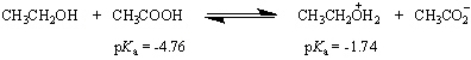
Correct Answer

verified
log10Keq = pKa(acid) - pK...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Which of the following energy diagrams represents the slowest reaction? 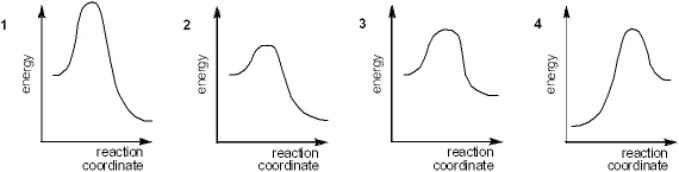
A) 1
B) 2
C) 3
D) 4
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Under which of the following conditions will a reaction be spontaneous when DH > 0?
A) TDS = 0
B) -TDS > DH
C) TDS < 0
D) DG = 0
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is a feature of a Brønsted-Lowry base?
A) proton donor
B) proton acceptor
C) electron pair donor
D) electron pair acceptor
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 75
Related Exams