A) Hydrogen bond
B) Water molecule
C) Oxygen atom
D) Hydrogen atom
E) Polar covalent bond
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
All of the following terms relate to lipids. Which does not belong with the other four?
A) Cholesterol
B) Estrogen
C) Steroid
D) Triglyceride
E) Bile salts
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In an x-ray film of the skeletal system, the dense tissue areas appear ________ because they ________ the x-rays; the less dense tissues appear ________ because they ________ the x-rays.
A) light; absorb; dark; do not absorb
B) dark; absorb; light; do not absorb
C) light; do not absorb; dark; absorb
D) dark; do not absorb; light; absorb
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In order to get energy (ATP) from food molecules in the final stage of respiration, humans require ________.
A) oxygen
B) sodium
C) carbon dioxide
D) ribose
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Electrolytes are substances that
A) form covalent bonds with water.
B) conduct electricity when dissolved in water.
C) cannot conduct electricity in solution.
D) are NOT found in the human body in any appreciable amounts.
E) are NOT charged particles.
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The mass of a chemical equal to its molecular weight in grams, containing 6.023 x 1023 molecules is a/an ________.
A) mole
B) molarity
C) ion
D) atomic mass unit
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Solution A has a pH of 10, and solution B has a pH of 2. Which of the following statements about these solutions is true?
A) Solution A and solution B are both basic.
B) Solution B is basic.
C) Solution A is acidic.
D) Solution B has a higher H+ concentration than solution A.
E) Solution A has a higher H+ concentration than solution B.
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Na (atomic no. 11) reacts with Cl (atomic no. 17) to become stable. In the reaction, Na will ________, while Cl will ________.
A) accept one electron; give up one electron
B) give up one proton; accept one proton
C) share one electron with chlorine; share one electron with sodium
D) become an anion; become a cation
E) give up one electron; accept one electron
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The molecule used most frequently by cells as a fuel belongs to which of the following groups?
A) Prostaglandins
B) Carbohydrates
C) Nucleic acids
D) Steroids
E) Phospholipids
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
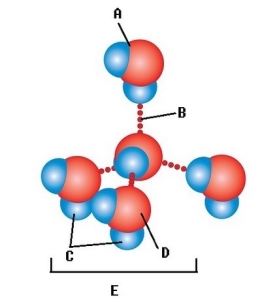 -Water accounts for 50% of the weight of a young adult female and 60% of a young adult male. What kind of bond is found at "A"?
-Water accounts for 50% of the weight of a young adult female and 60% of a young adult male. What kind of bond is found at "A"?
A) Hydrogen bond
B) Water molecule
C) Oxygen atom
D) Hydrogen atom
E) Polar covalent bond
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Normal pH range for blood is 7.35 to 7.45. If blood pH falls below 7.35,
A) an imbalance called alkalosis results.
B) nothing happens as this is an acceptable deviation.
C) an imbalance called acidosis results.
D) the blood becomes saltier.
E) the number of red blood cells decreases.
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Triglycerides are composed of
A) monosaccharides.
B) amino acids.
C) nucleotides.
D) glycerol and fatty acids.
E) None of the choices are correct.
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Arrange the following from largest to smallest: (1) Nucleus (2) DNA molecule (3) Skin cell (4) Chicken eggs
A) 1, 2, 3, 4
B) 4, 3, 1, 2
C) 3, 4, 2, 1
D) 2, 3, 1, 4
E) 4, 2, 3, 1
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A cation is
A) a combination of atoms held together by chemical bonds.
B) a positively charged ion.
C) a negatively charged ion.
D) a molecule that conducts electricity when placed in solution.
E) an alteration in the three-dimensional structure of a protein.
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Proteins
A) are the body's source of immediate energy.
B) are the building blocks of nucleotides.
C) provide much of the structure of body cells and tissues.
D) contain the genetic information of the cell.
E) insulate and cushion the body.
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Isotopes of the same element have
A) the same number of neutrons but different numbers of protons.
B) different numbers of protons and electrons.
C) the same mass number.
D) the same atomic number but differ in their mass numbers.
E) no mass number.
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following means a change in shape of a protein?
A) Amino acid
B) Peptide bond
C) Primary structure of protein
D) Secondary structure of protein
E) Denaturation
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
DNA
A) must travel to ribosomes to function.
B) contains the sugar deoxyribose.
C) is a single-stranded molecule.
D) is one of several amino acids.
E) assembles amino acids to make proteins.
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is a component of a nucleotide?
A) Adenine - a nitrogen base
B) Glucose - a monosaccharide
C) Cholesterol - a steroid
D) Calcium ions
E) ATP
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Inorganic chemists study substances ________ carbon, while organic chemists study substances ________ carbon.
A) lacking; containing
B) containing; lacking
C) containing more than 1 mole of; with less than a mole of
E) A) and C)
Correct Answer

verified
Correct Answer
verified
Showing 121 - 140 of 207
Related Exams