Correct Answer

verified
Structure 1 has the shortest bond length...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Which of the following best represents an sp2 hybridized atomic orbital of carbon which overlaps with the 1s atomic orbital of hydrogen to form a C−H σ bonding molecular orbital in ethene, H2C=CH2 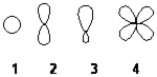
A) 1
B) 2
C) 3
D) 4
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following species possesses a formal charge?
A) CCl4
B) SiCl4
C) AlCl4
D) PCl3
F) B) and C)
Correct Answer

verified
Correct Answer
verified
True/False
Consider the structure of urea given below. 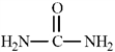 To complete the Lewis structure, six nonbonding electrons should be added, two to each of the nitrogen atoms and two to the oxygen atom.
To complete the Lewis structure, six nonbonding electrons should be added, two to each of the nitrogen atoms and two to the oxygen atom.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many electrons are there in the valence shell of the carbon atom of the methyl anion, CH3−?
A) 2
B) 4
C) 6
D) 8
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements is not true about the acetate anion, CH3CO2−?
A) The oxygen atoms bear the same amount of charge
B) The two carbon-oxygen bonds are the same length
C) The carbon atom bears the negative charge
D) It is basic
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements is not true?
A) The sp3C−H bond of an alkane is weaker than the spC−H bond of an alkyne.
B) The carbon-carbon triple bond of an alkyne is shorter than the carbon-carbon bond of alkenes.
C) The carbon-carbon triple bond of an alkene is exactly three times as strong as a carbon-carbon single bond of an alkane.
D) The sp3C−H bond of an alkane is longer than the spC−H bond of an alkyne.
F) A) and D)
Correct Answer

verified
Correct Answer
verified
True/False
The following two structural formulas represent isomers. | |
B) False
Correct Answer

verified
Correct Answer
verified
Short Answer
The following molecule is classified as a __________amine.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following molecules has a molecular dipole moment? 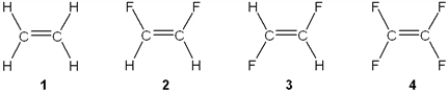
A) 1
B) 2
C) 3
D) 4
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following molecules has a molecular dipole moment? 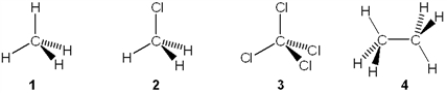
A) 1
B) 2
C) 3
D) 4
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following bonds is the most polar?
A) O−H
B) C−H
C) C−C
D) H−H
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the approximate C−C−C bond angle in propyne, HC≡CCH3?
A) 90°
B) 109°
C) 120°
D) 180°
F) C) and D)
Correct Answer

verified
Correct Answer
verified
True/False
In drawing the Lewis structure for an organic compound, the carbon atoms should always be shown with eight total electrons.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following bonds has the smallest dipole moment?
A) C−N
B) C−O
C) C−F
D) O−H
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the ground-state electronic configuration of a fluorine atom (fluorine: atomic number 9) ?
A) 1s12s12p7
B) 1s22s22p5
C) 1s22s22p6
D) 1s02s22p7
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Essay
What is the molecular formula of Ritalin, shown below? 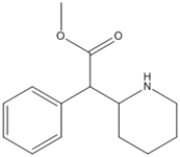
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is a tertiary alcohol?
A) CH3CH2OCH3
B) (CH3) 3COH
C) (CH3) 2CHOH
D) CH3CH2CH2OH
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following bonds is a polar covalent bond?
A) Na−Cl
B) C−Cl
C) C−H
D) Cl−Cl
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Fill the appropriate electronic configuration in the blank. K (1s22s22p63s23p64s1) + Cl (1s22s22p63s23p5) → K+ _____ + Cl- (1s22s22p63s23p6)
A) (1s22s22p63s23p64s2)
B) (1s22s22p63s23p65s1)
C) (1s22s22p63s23p6)
D) (1s22s22p63s23p64s1)
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 118
Related Exams