B) False
Correct Answer

verified
Correct Answer
verified
Essay
What is the major organic product obtained from the following reaction? 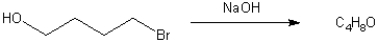
Correct Answer

verified
11ead7c8_9da8_fc3c_84b0_1d986d74c469_TB7078_00
Correct Answer
verified
Multiple Choice
What is the best choice of reagent to perform the following transformation? 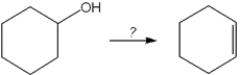
A) H2SO4
B) H2O
C) NaOCH3
D) KOtBu
F) A) and C)
Correct Answer

verified
A
Correct Answer
verified
Multiple Choice
Which of the following anions is the most nucleophilic in polar protic solvents?
A) F−
B) Cl−
C) Br−
D) I−
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Essay
Draw all of the chloroalkanes that undergo base-promoted dehydrochlorination to form the following alkene? 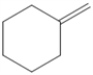
Correct Answer

verified
Correct Answer
verified
Essay
What is the major organic product obtained from the following reaction? 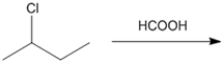
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the equation for the rate of formation of 2-methoxypropane, CH3CH(OCH3) CH3, from the reaction of 2-bromopropane (i-PrBr) with methanol?
A) Rate = k [i-PrBr]
B) Rate = k [i-PrBr]2
C) Rate = k [CH3OH]
D) Rate = k [i-PrBr][CH3OH]
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements is not true regarding SN2 reactions?
A) A carbocation intermediate is formed.
B) The mechanism has only one step.
C) Aprotic solvents are good choices for SN1 reactions.
D) The stereochemical outcome is inversion at the carbon bearing the leaving group.
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Essay
Draw all of the chloroalkanes that undergo base-promoted dehydrochlorination to form the following alkene? 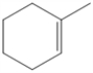
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements related to SN1 reactions is not true?
A) The heterolysis of a bond between atoms which do not bear formal charges always produces a cation and an anion
B) The charged carbon atom of a carbocation has a complete octet of valence shell electrons
C) Carbocations are Lewis acids
D) Nucleophiles seek centers of low electron density
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the equation for the rate of formation of 2-methoxypropane, (CH3CH(OCH3) CH3, from the reaction of 2-bromopropane (i-PrBr) with sodium methoxide (NaOCH3) ?
A) Rate = k [i-PrBr]
B) Rate = k [i-PrBr]2
C) Rate = k [NaOCH3]
D) Rate = k [i-PrBr][NaOCH3]
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following represents the transition state of the rate-determining step in the reaction between 2-chloropropane and sodium amide leading to elimination? 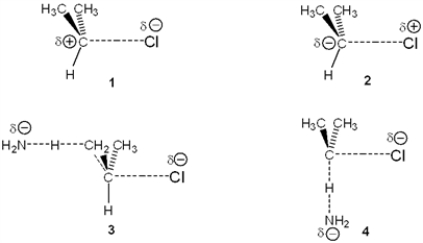
A) 1
B) 2
C) 3
D) 4
F) A) and B)
Correct Answer

verified
C
Correct Answer
verified
Multiple Choice
What is the major product formed upon treatment of (R) 2-bromohexane with sodium cyanide?
A) (R) 2-cyanohexane
B) (S) 2-cyanohexane
C) 1-hexene
D) 2-hexene
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the major organic product obtained from the following reaction? 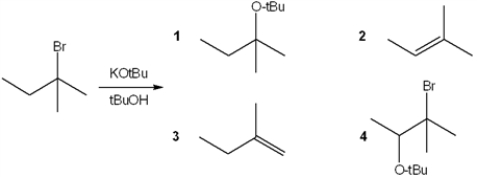
A) 1
B) 2
C) 3
D) 4
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the equation for the rate of formation of 1-iodobutane from the reaction of 1-chlorobutane (BuCl) with NaI by an SN2 mechanism?
A) Rate = k [BuCl]
B) Rate = k [BuCl][NaI]
C) Rate = k [NaI]
D) Rate = k [BuCl]2
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is most likely to undergo rearrangement during reaction with methanol? 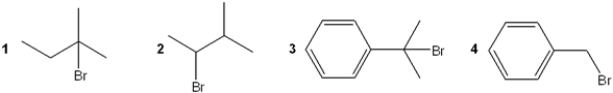
A) 1
B) 2
C) 3
D) 4
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following represents the transition state of the reaction between methyl iodide and ammonia? 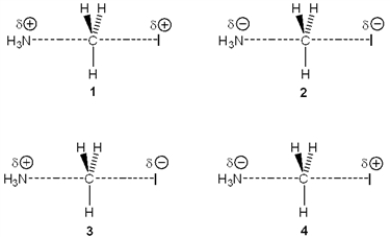
A) 1
B) 2
C) 3
D) 4
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds undergoes the most rapid hydrolysis reaction? 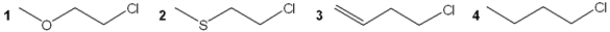
A) 1
B) 2
C) 3
D) 4
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the major organic product(s) obtained from the following reaction? 
A) 1-hexyne
B) 2-hexyne
C) propene + propyne
D) propane + propyne
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the major organic product obtained from the following reaction? 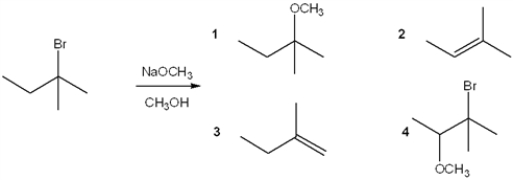
A) 1
B) 2
C) 3
D) 4
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 104
Related Exams