A) 90°
B) 109.5°
C) 120°
D) 180°
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider compounds which contain both a heteroatom and a double bond.For which compound is no additional Lewis structure possible? 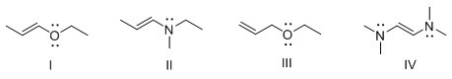
A) I
B) II
C) III
D) IV
F) B) and D)
Correct Answer

verified
C
Correct Answer
verified
Multiple Choice
Which of the following Lewis structures is correct? 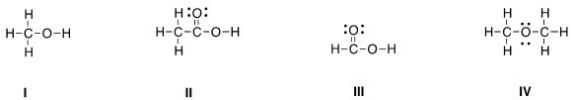
A) I
B) II
C) III
D) IV
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following resonance structures is the least important contributor to the resonance hybrid of the formate anion,HCOO-? 
A) I
B) II
C) III
D) IV
F) B) and C)
Correct Answer

verified
B
Correct Answer
verified
Multiple Choice
Which molecule has the greatest difference in electronegativity (DE) between the two different elements?
A) CO2
B) H2S
C) NH3
D) H2O
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many constitutional isomers are there for a molecule having the molecular formula C3H6?
A) 1
B) 2
C) 3
D) 4
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following molecules has a net dipole moment of zero? 
A) I
B) II
C) III
D) IV
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds has an atom with more than eight valence electrons?
A) H2CO3
B) H2SO4
C) H2O
D) HBr
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Follow the curved arrows to draw the second resonance structure for the ion below. 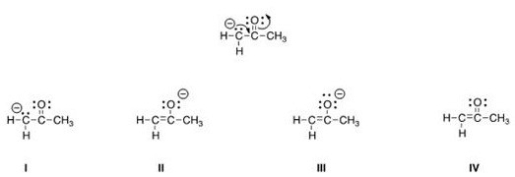
A) I
B) II
C) III
D) IV
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the formal charge of carbon in carbon monoxide (CO) when drawn with a triple bond?
A) 0
B) -2
C) -1
D) +1
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which atomic orbitals overlap to form the C-H s bonding molecular orbitals of acetylene,C2H2?
A) Csp + H1s
B) C2p +H1s
C) Csp3 + H1s
D) Csp2 + H1s
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the molecular geometry around the boron atom in BH3?
A) Tetrahedral
B) Trigonal Planar
C) Trigonal Pyramidal
D) Linear
F) C) and D)
Correct Answer

verified
B
Correct Answer
verified
Multiple Choice
Which of the following covalent bonds has the largest dipole moment?
A) C-H
B) C-C
C) C-O
D) H-F
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What 2 things will change between two resonance structures?
A) The position of multiple bonds and non-bonded electrons.
B) The position of multiple bonds and single bonds.
C) The placement of atoms and single bonds.
D) The placement of atoms and non-bonded electrons.
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is the appropriate conversion of (CH3) 2CHCH2CHClCH3 to a skeletal structure? 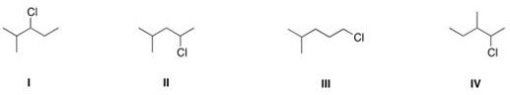
A) I
B) II
C) III
D) IV
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which is not an acceptable Lewis structure for the anion CH2NCO-? 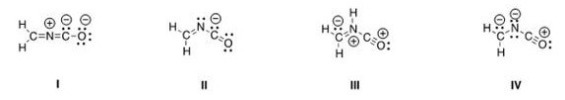
A) I
B) II
C) III
D) IV
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the ground-state electronic configuration of a magnesium cation (Mg2+) ?
A) 1s2,2s2,2p6
B) 1s2,2s2,2p6,3s1
C) 1s2,2s2,2p6,3s2
D) 1s2,2s2,2p6,3s2,3p2
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which is more important in each pair of contributing resonance structures? 
A) II,IV,V
B) II,III,V
C) II,III,VI
D) I,IV,V
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following molecules are constitutional isomers? 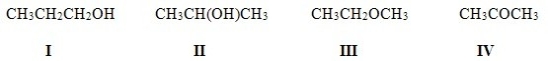
A) I,II,IV
B) II,III,IV
C) I,III,IV
D) I,II,III
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the ground-state electronic configuration of a fluorine atom?
A) 1s2,2s2,2p2
B) 1s2,2s2,2p3
C) 1s2,2s2,2p4
D) 1s2,2s2,2p5
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 77
Related Exams